-
The Philippine Food and Drug Administration (FDA) is warning the public about unregistered and fake tocilizumab drugs.
This is worrisome because tocilizumab is currently one of the drugs doctors use to treat the sickest patients with COVID-19 in hospitals. Tocilizumab has an emergency use authorization (EUA) from the U.S. FDA last June 24, 2021, as “an addition to the routine care patients receive for treatment of COVID-19.”
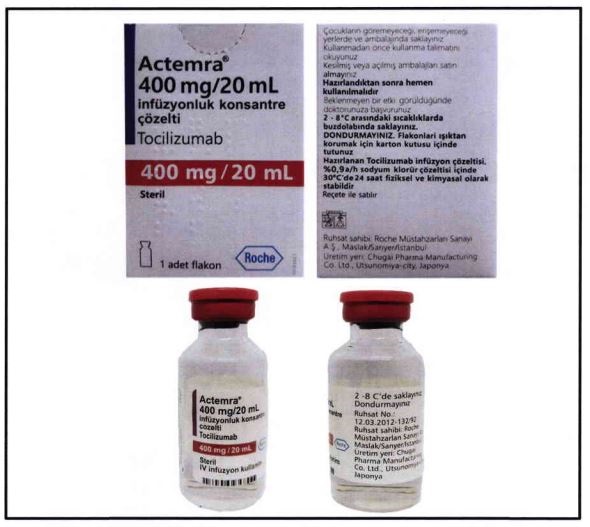
The lcoal FDA’s health warning is against the purchase and use of one of the tocilizumab’s brands, Actemra, specifically the 400 mg/20 mL with label in foreign language.
This particular brand has not yet undergone the registration process and obtained the proper authorization from our FDA. PH FDAThe government agency reiterates in its advisory it cannot guarantee the safety and quality of unregistered drug products.
How to spot fake Tocilizumab (Actemra)
Unfortunately, before Actemra even gets FDA authorization, Actemra counterfeit products have penetrated the market. FDA issued guidelines on how to spot one via the image below.
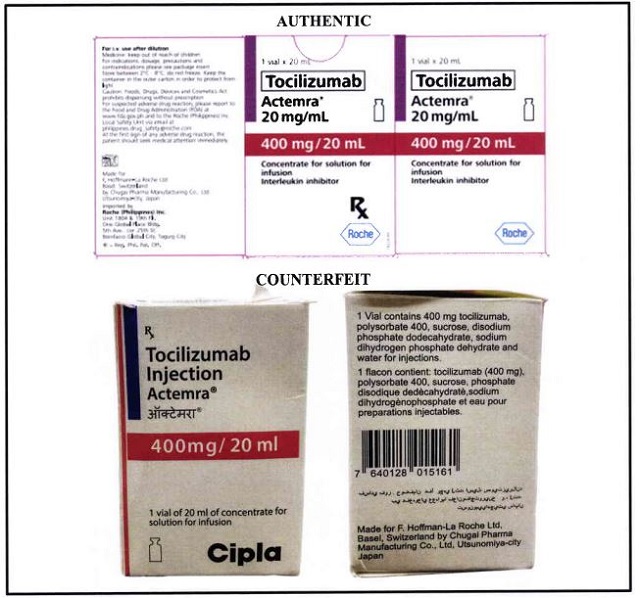
ADVERTISEMENT – CONTINUE READING BELOWThe authentic Tocilizumab Injection Actemra 400 mg/ 20 mL has a thick violet line on the edge of its box, while the counterfeit one has a thick blue line.
The authentic Actemra has a vial symbol opposite the dosage. The counterfeit vial is placed at the bottom left corner. Roche is also the manufacturer of Actemra. PH FDAMeanwhile, the counterfeit box features text in a non-English language below the product’s name and after the bar code. The two also differ in the text and labels seen on the lower front side of the box.
The name “Cipla” is a giveaway that this is fake. Roche is the pharma company that manufactures this particular brand with the generic name tocilizumab. PH FDACONTINUE READING BELOWRecommended VideosThe vial of the counterfeit product will look like the image above. It still contains the thick blue line on its sticker label, which is one of the indicators seen on the counterfeit box. The Cipla label is also present in its sticker label beside the dosage, another indicator seen on the fake box.
Tocilizumab is often used to treat moderate to severe rheumatoid arthritis. But the “lab-generated antibody” shows potential in blocking “the interleukin-6 pathway, which can cause inflammation during COVID-19 and other diseases.” It has also been shown to benefit patients with high levels of inflammation in early results from another trial.
Read here to know more about the treatments helping COVID-19 patients survive.
FDA Warns Of Unregistered And Fake Tocilizumab, Which Is Used In COVID-19 Treatment
Source: Progress Pinas

0 Comments